When considering adhesives, bonding surface is a critical factor. Unlike processes that alter and join surfaces, such as welding, bonding with adhesives involves attaching two surfaces, without changing their individual characteristics. That being the case, the adhesive selected must be appropriate for the surfaces involved in the application.
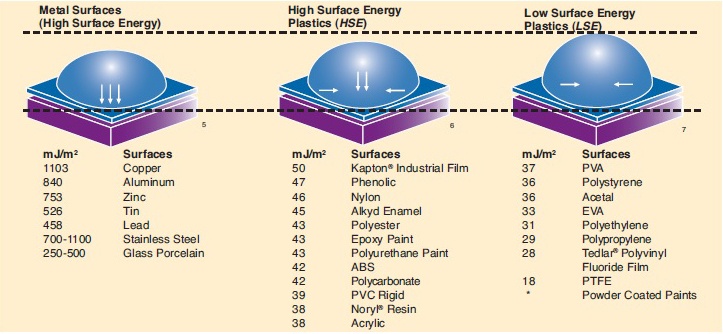
Surface energy is a term used to describe the surface of a given substrate; surface energies range from high to low. The molecular force of attraction between unlike materials determines their adhesion. The strength of attraction is depends on the surface energy of the substrate. High surface energy means a strong molecular attraction, while low surface energy means weaker attractive forces.
To understand the concept of surface energy, consider water on the un-waxed hood of a car. The hood that has not been waxed has high surface energy, so water puddles on the surface of the hood. A waxed hood, on the other hand, has low surface energy, so water beads up instead of flowing out. As with water, adhesive on a high surface energy surface flows and “wets out” the surface; wetting out is necessary to form a strong bond.
Many adhesives are specifically formulated for low surface energy surfaces. The 3M chart shown below demonstrates surface rankings and illustrates the concept of relative surface energy. Whatever the surface energy of a given substrate may be, the area of attachment must be unified, dry, and clean to maximize adhesive contact.
Can-Do Tape has been a Master distributor of adhesive tape products for over 30 years. Our professionally trained team is qualified to assist you in determining the right product for your application.

